Kupfer/Zink Daniell-Element mit Halbzelle
by B.Lachner - uploaded on October 11, 2015, 6:20 pm
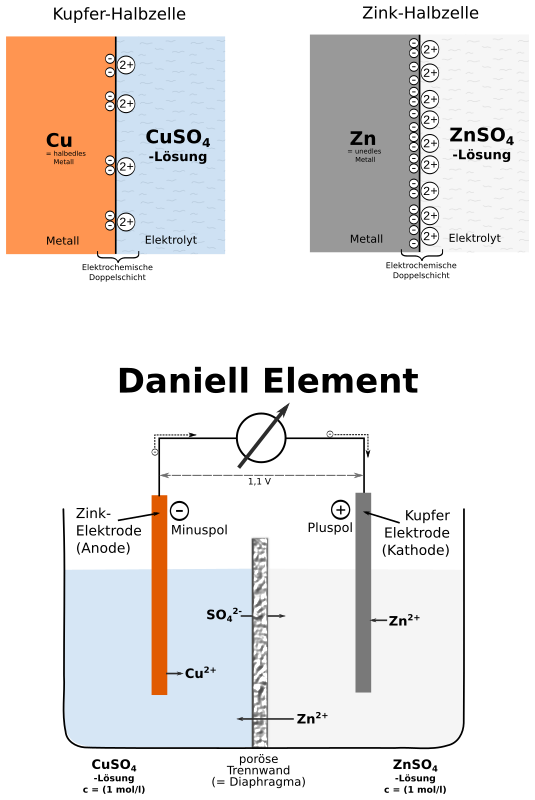
The Daniell cell is also the historical basis for the contemporary definition of the volt, which is the unit of electromotive force.
In the Daniell cell, copper and zinc electrodes are immersed in a solution of copper(II) sulfate and zinc sulfate respectively. At the anode, zinc is oxidized.
The Daniell cell is a type of electrochemical cell invented in 1836 by John Frederic Daniell, a British chemist and meteorologist, and consisted of a copper pot filled with a copper sulfate solution, in which was immersed an unglazed earthenware container filled with sulfuric acid and a zinc electrode.
Log into OpenClipart
- Tags
- Batterie Battery Chemie Chemie-Theorie Chemistry Copper Electricity Elektrochemie Halbzelle Ion Ionen Ions Kupfer Lösung Solution Strom Zinc Zink
- Safe for Work?
- Yes
0 Comments. Please login to comment or add your own remix.